Advertisment
How much contamination is too much in the workplace?

Working with HMPs calls for safe systems to minimise the risks of occupational exposure for staff. However, monitoring of contamination must also be undertaken to check that systems are working, according to Birgit Tans, Specialist Compounding Pharmacist, University Hospitals Leuven and Paul Sessink, Managing Director, Exposure Control Sweden AB.
Healthcare workers can be exposed to hazardous medicinal products (HMPs) via two main routes – inhalation, when powders are handled, or absorption via the skin, usually after contact with contaminated surfaces. The second of these is the more common route and this is the reason why protective gloves are required.
When working with HMPs, “everything you can touch can be contaminated ….. so that’s the reason why you have always to wear … protective gloves … because when it’s on your hands it can get into your body”, explains Dr Sessink.
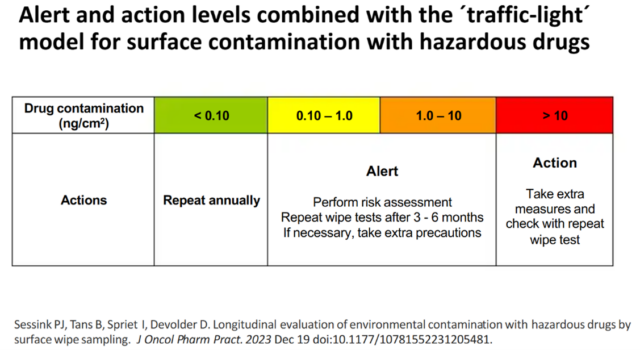
Contamination with HMPs is measured by surface wipe sampling. This technique involves swabbing a defined area with a suitable liquid (e.g. using the wipe kit from Exposure Control) to collect the drug residues. After analysis the amount of drug in the swab can then be expressed as nanograms per square centimetre (ng/cm2).
The results can be interpreted using a ‘traffic lights’ model. “It gives you a first impression about the contamination and your working conditions. If it’s all low or ‘not detectable’, you have a good working procedure, all is fine. When it’s high, then there are probably spills and leakage and, …. according to the results you can make changes in your process or in the devices you are using”, he says.
Safe systems
One of the main reasons for having safe systems of working is to prevent contamination of the working environment with hazardous drugs and thereby prevent exposure of healthcare workers. Failure to prevent contamination in the first place just creates a lot of extra work in removing contamination, explained Dr Sessink. His own work, using wipe tests inside isolators and biohazard safety cabinets, has shown that HMPs are often present, indicating that spillages and leaks have occurred. “The point is, as long as it stays inside and is not getting out on a product, like an infusion bag or a syringe or whatever, then it’s fine – but there is never a guarantee that [a product] is clean when it’s leaving the pharmacy and going to nursing [areas]”, he says. The way to avoid this problem is to use closed system transfer devices (CSTDs) for the preparation process. “If you have that process closed and there is no spill or leakage, you will not have a contaminated product and you will not spread the contamination – so I would say 90-95% of the problem can be solved there”, says Dr Sessink. The other source of contamination is drug vials that are contaminated on the outside when they arrive from the manufacturer. Dr Sessink’s advice is to specify that vials must be free of exterior contamination when they are purchased. “If you cover that clean vial issue and you cover the potential leakage by using CSTDs, almost the whole issue of contamination, aside from accidents, because they can always happen. Aside from accidents, the normal process can be 100% controlled”, he says.
Birgit Tans describes the measures that make up the safe systems of working at the Pharmacy Department in the Leuven University Hospitals. The people involved in compounding of HMPS wear gowns, gloves and other personal protective equipment (PPE) and work in BSCs (biohazard safety cabinets to ensure that they do not come into contact with the drugs. In addition, CSTDs are used to prevent the release of drug aerosols when drugs are transferred from vials to infusion bags. Because the devices are needle-free, needle-stick injuries are also avoided. The work is carried out following standard operating procedures and regular education and training is provided for the staff, she emphasises.
About Birgit Tans and Paul Sessink
Birgit Tans is a hospital pharmacist at the University Hospitals of Leuven in Belgium. For the past 30 years she has specialised in compounding of cytotoxic drugs and is an expert on safe handling of hazardous drugs.
Paul Sessink first studied chemistry and later completed a PhD on hazardous drug exposure. In 1995 he founded the company, Exposure Control to provide services related to monitoring of hazardous drugs in the working environment. The company has provided services to about 350 hospitals in the world.
 |
 |
Read and watch the full series on our website or on YouTube.





