Advertisment
US drug approvals 2010-2019 align with US, but not global, burden of disease; expedited approval programs may make the gap worse

Drug approvals in the United States between 2010-2019 were aligned with the US, but not global, burden of disease and the increasing number of expedited drug approvals could make the gap worse according to a study in the BMJ Open. The study also demonstrates that drugs indicated for conditions with the greatest burden of disease were less likely to be approved through the FDA’s expedited approval programs that reduce the timeline and cost of drug development, thus making it relatively more expensive to develop these products. US markets and FDA approval play an important role in shaping the product portfolios of global pharmaceutical companies; as such, expedited approval programs may inadvertently disincentivize development of drugs for conditions associated with greatest burden of disease.
The study from Bentley University’s Center for Integration of Science and Industry titled “Association between expedited review designations and the US or global burden of disease for drugs approved by the US Food and Drug Administration 2010-2019: a cross-sectional analysis” used metrics developed by the World Health Organization (WHO) to measure the years of life lost (YLL), years of healthy life lost with disability or ill health (YLD), and total disease burden (disability-adjusted life years, DALYs) associated with the conditions treated by the 387 drugs approved by the FDA from 2010-2019.
This research showed that the majority of drug approvals were indicated for conditions in the highest quartile of US disease burden and years of life lost. In contrast, only a quarter of these approvals were indicated for conditions in the highest quartile of global disease burden or years of healthy life lost with disability or ill health. There was no alignment between the indications for approved drugs and either US or global disability. Since the US burden of disease represents <4% of the global disease burden, these data indicate that there is little alignment between the drugs being developed by the global pharmaceutical industry and the health needs of global human populations.
The four expedited review programs in the US – fast track, breakthrough therapy, accelerated approval, and priority review – are each designed to reduce the timelines or costs of developing products for selected “serious” diseases and have been widely adopted by industry. The present study shows that the likelihood of a drug receiving one or more designations for expedited approval was lower for products indicated for conditions with greater burden of disease in US or global populations and was dramatically lower for products targeting the greatest years of healthy life lost with disability or ill health.
“Product development by global pharmaceutical companies is strongly influenced by potential sales in US markets, and companies typically see little commercial opportunity in developing products for diseases that primarily afflict low- or middle-income countries” said Dr. Fred Ledley, Director of the Center for Integration of Science and Industry and Professor of Natural & Applied Sciences and Management at Bentley University. “If expedited review programs disproportionately reduce the cost of developing products indicated for conditions with lower disease burden, this could inadvertently disincentivize development of products that address the greatest burden of human disease.”
This paper is one of a series from the Center for Integration of Science and Industry examining the public sector investment and impacts of drugs approved by the FDA from 2010-2019.
Dr. Matthew Jackson, a former fellow at the Center for Integration of Science and Industry, was the lead author of this study along with Dr. Gregory Vaughan, Associate Professor of Mathematical Sciences, and Dr. Ledley.
This work was supported by grants from the Institute for New Economic Thinking and National Biomedical Research Foundation to Bentley University.
THE CENTER FOR INTEGRATION OF SCIENCE AND INDUSTRY at Bentley University focuses on advancing the translation of scientific discoveries to create public value. The Center is an environment for interdisciplinary scholarship spanning science, data analytics, business, and public policy. For more information, visit www.bentley.edu/sciindustry and follow us on Twitter/X @sciindustry and LinkedIn. The Center for Integration of Science and Industry is an affiliate of the Center for Health and Business at Bentley University.
BENTLEY UNIVERSITY is one of the nation’s top business schools and a lifelong-learning community that creates successful leaders who make business a force for positive change. With a combination of business and the arts and sciences and a flexible, personalized approach to education, Bentley provides students with critical thinking and practical skills that prepare them to lead successful, rewarding careers. Founded in 1917, the university enrolls >4,000 undergraduate, graduate, or PhD students and is set on 163 acres in Waltham, Massachusetts, 10 miles west of Boston. For more information, visit bentley.edu. Follow us on Twitter/X @BentleyU #BentleyUResearch.
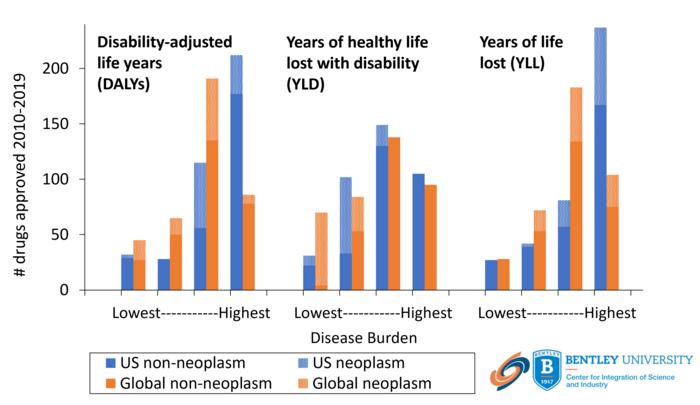
IMAGE: NUMBER OF FDA DRUG APPROVALS 2010-2019 FOR CONDITIONS RANKED BY LOWEST-HIGHEST US (BLUE) OR GLOBAL (ORANGE) BURDEN OF DISEASE. THE BURDEN OF DISEASE IS MEASURED BY DALYS, YLL OR YLD. THE NUMBER OF FDA APPROVALS IS SHOWN FOR NEOPLASTIC (PATTERNED FILL) AND NON-NEOPLASTIC (SOLID FILL) CONDITIONS.
view more CREDIT: BENTLEY UNIVERSITY





